New Drugs Show Promise for Treating Geographic Atrophy
Written By: BrightFocus Editorial Staff
Written By: BrightFocus Editorial Staff
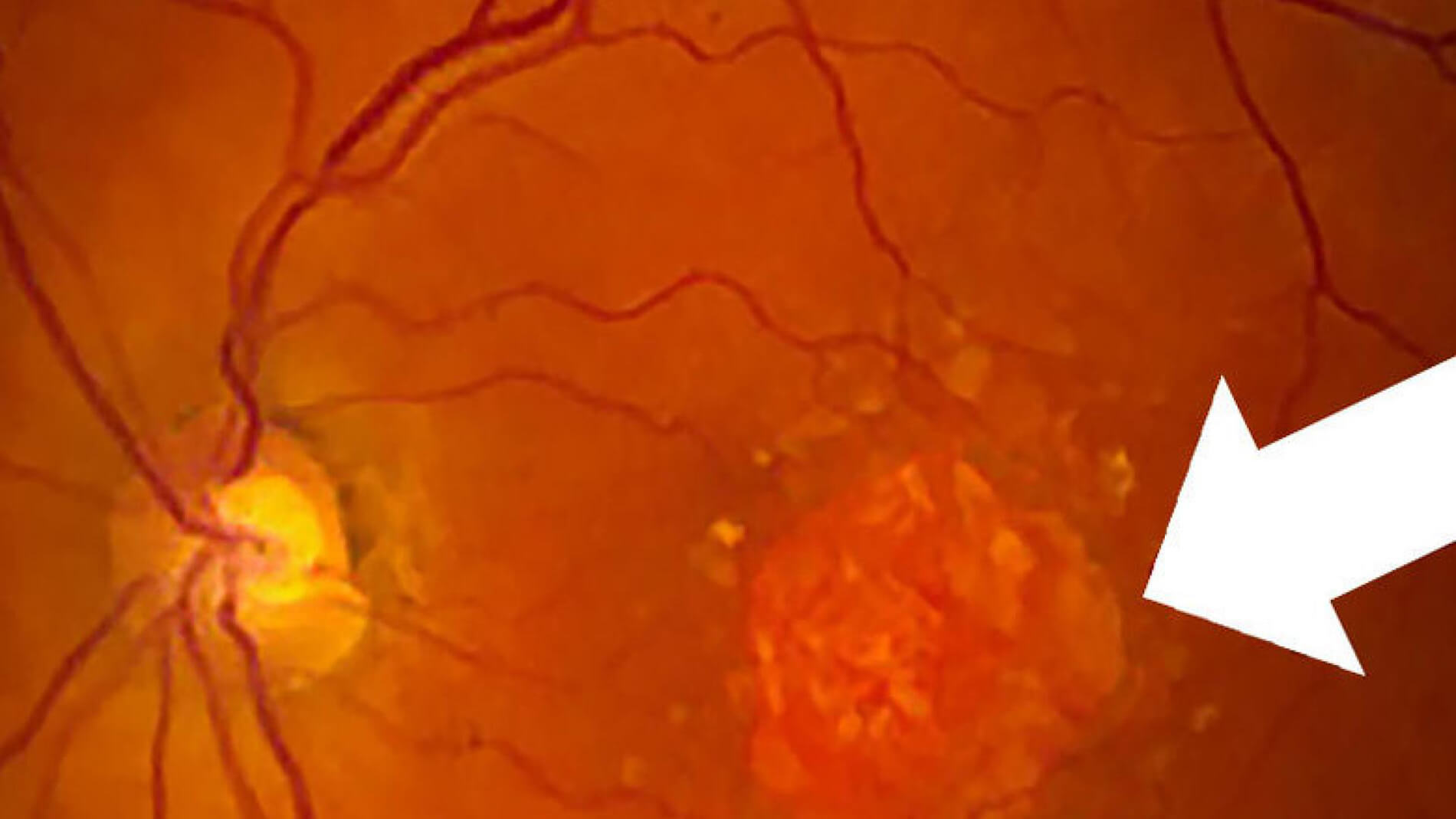
In advanced stages, age-related macular degeneration (AMD) can take two very different forms: It can become wet AMD, where fragile, leaky blood vessels invade the retinal space, or it can take the form of geographic atrophy (GA), where patches of retinal cells waste away and die. Both types of advanced AMD may cause loss of central or straight-ahead vision. However, whereas treatments for wet AMD have been available for decades, effective treatments are still needed for GA. Today there’s new hope, as several potential GA drugs are being tested in clinical trials.
A promising group of drugs known as complement inhibitors treats GA by quieting the immune response. A new oral complement inhibitor, danicopan (ALXN2040), is being developed by Alexion Pharmaceuticals, Inc., a subsidiary of Astra-Zeneca, and last year entered early stages of clinical testing for use in GA. There are other complement inhibitors further along in clinical development as well.
Moshe Vardi, MD, vice president and Global Medicine Team Lead at Alexion, and Aleksander Skuban, MD, Executive Medical Director for Neurology and Ophthalmology Clinical Development and Translational Sciences at Alexion, offer insights on how danicopan works and provide details about their clinical trial.
The following is an edited version of a BrightFocus Chat featuring Drs. Vardi and Skuban. Listen to the full audio recording of this Chat or read the transcript.

BrightFocus: Why are there treatments for wet AMD and none yet for geographic atrophy?
Dr. Moshe Vardi, Alexion: Both diseases are late-stage forms of AMD, but very distinct in the way that they develop. Wet AMD is primarily thought to be driven by destruction of blood vessels, while GA is primarily driven by an immune response which is mediated by the complement system. So, different diseases, and different perspectives on how to treat them.
BrightFocus: Is the complement system part of the immune system?
Dr. Vardi: You can think about the complement system as a first line of defense in our body and obviously in the eyes as well. It’s always active, and able to quickly respond to insults, meaning that if there is an identification of something that is wrong, the complement system kicks into play and pulls in the rest of the immune system.
In diseases such as AMD, this complement system is going out of control. It is working more than it needs to work, and this is the source of the pathology in AMD. We are learning from clinical studies that when we inhibit the complement system, which is hyper-reactive, we get good outcomes.
BrightFocus: What would danicopan do to protect or help an eye that has GA?
Dr. Aleksandar Skuban, Alexion: Our hypothesis is that danicopan slows the progression of GA over time, and that it’s safe and effective to use in the population.
BrightFocus: Is it an injection or a pill?
Dr. Skuban: This is a pill— or actually a tablet—that would be administered orally.
BrightFocus: Have clinical trials begun?
Dr. Skuban: We have a phase 2 proof-of-concept study that will be an assessment of how danicopan works in GA. We are, I believe, at the beginning of a very exciting scientific clinical journey.
BrightFocus: Can you help us understand the different phases of clinical trials and where danicopan is in that process?
Dr. Vardi: There’s a very robust process to develop new drugs and to get them to patients, and that includes different phases.
Phase 1 is where you test drugs on primarily healthy volunteers to understand the initial safety, as well as some pharmacological-related attributes of the drug.
In phase 2, the focus is on whether or not it has an effect, what is the right dose, and are there any safety concerns. This is the stage that danicopan is currently in for GA.
Phase 3 studies are confirmatory studies, again focused on efficacy and safety, and serve as the bigger data source for regulators, such as the FDA, to assess the balance between effectiveness and safety and then decide whether they are granting approval for the drug or not.
BrightFocus: How long can this process take?
Dr. Vardi: It usually takes from seven to up to 10 years to take a drug from phase 1 all the way through approval. It’s a very long process. The good news is that there are other companies with other complement inhibitors that are further along in the process.

BrightFocus: What type of person would be eligible to participate in your danicopan trial? Would they have GA in one eye or both?
Dr. Skuban: The presence of a qualifying GA change in one eye would be sufficient for the patient to qualify. There does not need to be GA in both eyes.
BrightFocus: How would someone go about learning how to participate in this and other clinical trials for geographic atrophy?
Dr. Skuban: The patient’s physician, ophthalmologist, or retinal specialist may be an excellent resource. This study is listed on the ClinicalTrials.gov website, and our team is working on a study-specific website that will contain more information.

Download Clinical Trials: Your Questions Answered, a BrightFocus brochure that includes questions to ask the research team before enrolling in a clinical trial. You can also request to have a free copy of the brochure mailed to you by calling BrightFocus at 1-800-437-2423.
BrightFocus: Do you anticipate that danicopan will be effective for geography atrophy exclusively, or possibly also for wet AMD?
Dr. Vardi: We are testing danicopan for GA. This is where the science leads us. There is some proof that complement is part of the dysregulation in wet AMD, as well, but that needs further assessment in scientific research.
At the end of the day, the drug will work on both eyes even though, in some cases, only one eye will qualify for the study. We do expect to have patients with dry AMD in the qualifying eye and potentially wet AMD in the other eye, so we will generate data that would be very interesting for us and also for the scientific community – to see how a systemic complement inhibitor, in this case, may affect wet AMD.
BrightFocus: How does early-stage research — also called “basic” research — figure into drug development?
Dr. Vardi: Science is where concepts end up being tested, hypotheses generated, and then we — in the drug development space — take these ideas and move them forward. But basic science is where it all starts.
In order for us to think that danicopan could work in geographic atrophy, we had to rely on a lot of science that was generated by organizations, such as BrightFocus. I want to thank BrightFocus for their investment in areas in which so much unmet need exists.
In order for us to think that danicopan could work in geographic atrophy, we had to rely on a lot of science that was generated by organizations, such as BrightFocus. I want to thank BrightFocus for their investment in areas in which so much unmet need exists.
Dr. Moshe Vardi
BrightFocus: Are there any other thoughts you’d like to share about what’s ahead?
Dr. Vardi: In GA, there are no current available treatments, but there is a lot going on in research and development, and we are very hopeful that in the near to intermediate future, there will be something for the treatment of geographic atrophy.
Dr. Skuban: It is a very promising field. We are really happy to have been able to share some of that today.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
Help Fight Macular Degeneration and Save Sight
Your donation helps fund critical research to bring us closer to a cure for this sight-stealing disease and provide vital information to the public.
Donate Today