Discoveries Update Tau’s Role in Neurodegeneration, Suggest New Drug Target
Written By: BrightFocus Editorial Staff
Written By: BrightFocus Editorial Staff
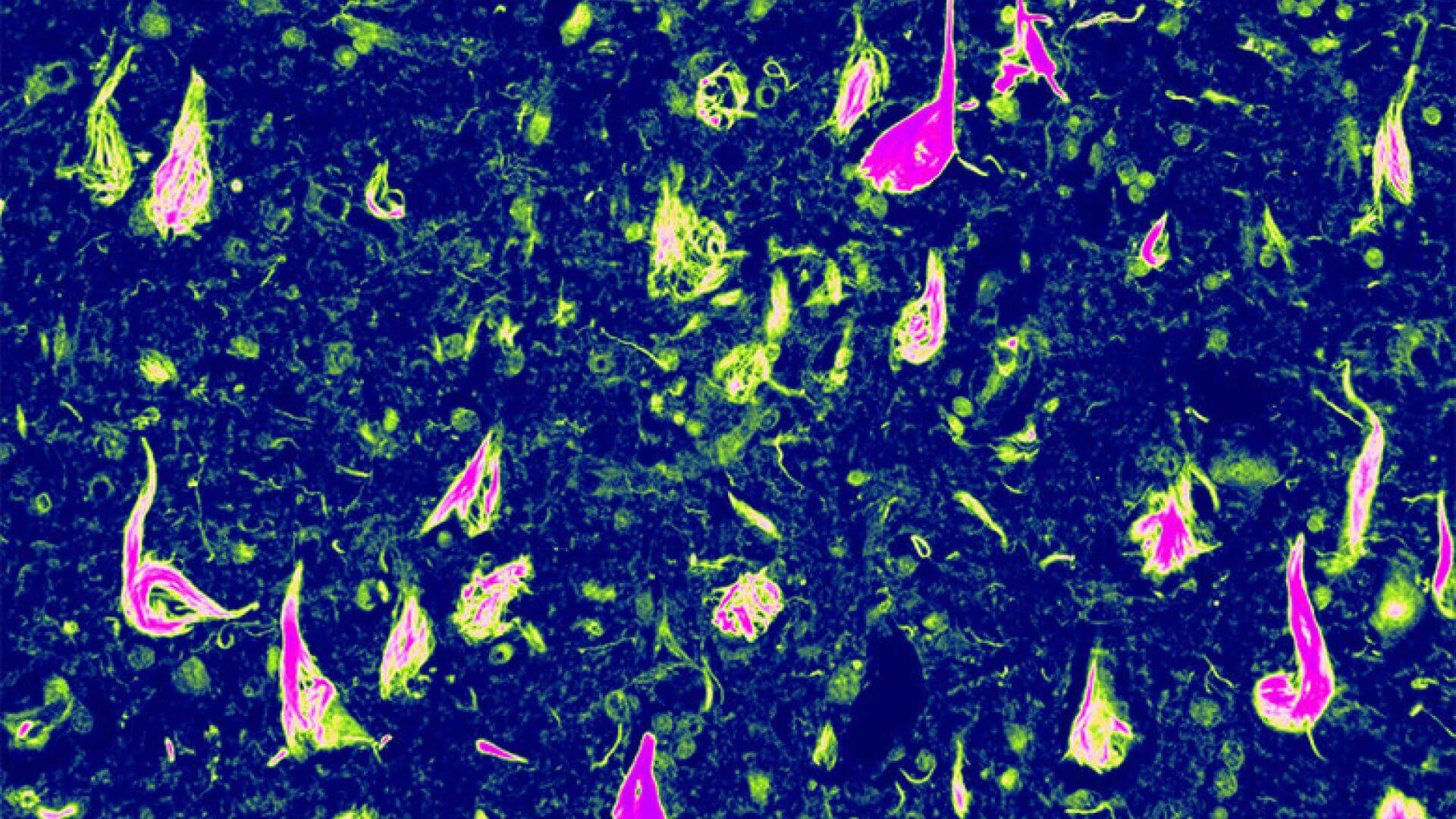
What: Newly discovered genetic mutations, and a new model capable of mimicking tau spread in humans, elucidate mechanisms by which tau contributes to neurodegeneration. This knowledge opens up new avenues for Alzheimer’s drug development.
Where: Darwich, NF, et al, “Autosomal Dominant VCP Hypomorph Mutation Impairs Disaggregation of PHF-Tau,” Science, 2020; Weitzman SA et al, “Insoluble Tau From Human FTDP-17 Cases Exhibit Unique Transmission Properties In Vivo,” Journal of Neuropathology & Experimental Neurology, 2020; and He Z, et al. Transmission of tauopathy strains is independent of their isoform composition. Nature Communications, 2020.
BrightFocus Connection: This research was supported in part by an Alzheimer’s Disease Research grant to coauthor Zhuohao He, PhD, while he was at the University of Pennsylvania. He is now a principal investigator with the Chinese Academy of Sciences, Shanghai.
Why It Is Important: Tau proteins undergo molecular changes in Alzheimer’s disease (AD) and other neurodegenerative diseases, causing them to misfold and accumulate in neurofibrillary tangles (eg, tau tangles) that destroy brain cells. Researchers are working to better understand this process.
Now an international group of scientists, working collaboratively, have identified a rare, inheritable genetic mutation that is associated with a new type of dementia linked to neurofibrillary “tau tangles.” The genetic studies leading to the discovery were done on two families, one in Greece and the other in the United States, both with a novel mutation associated with frontotemporal degeneration, an AD-associated form of neurodegeneration. Interestingly, neither family had a mutation on the microtubule associated protein tau (MAPT) gene that encodes for tau, but on a related gene, the valosin-containing protein gene (VCP) that encodes proteins associated with tau breakdown and disposal. Typically, mutations linked to AD-type neurodegeneration affect the production or aggregation of misfolded tau, and less is known about genetic factors impeding tau clearance. These recent findings represent a breakthrough by suggesting that impaired tau turnover can promote neurofibrillary formation (or tangle formation), highlighting VCP as a potential new drug target to prevent tau accumulation and cell death.

As coauthor and contributor to this work, BrightFocus grantee Zhuohao He, PhD, is a senior research investigator within the Center for Neurodegenerative Disease Research at Penn’s Perelman School of Medicine. The center is directed by Virginia Lee, PhD, a BrightFocus supporter and winner of a major award for scientific breakthroughs. Dr. He’s ADR project seeks to identify the specific strains of tau that accumulate in human AD, and to distinguish them from other tau proteins found in brain cells, in order to track the initial deposit and spread of tau in AD’s onset. To study this, he developed an animal model with the human versions of tau protein.
A second paper he recently coauthored focuses on the MAPT gene, and the discovery of rare MAPT mutations associated with frontotemporal dementia with parkinsonism-17 (FTDP-17), another tau-associated neurodegenerative disease. It reports on the first experimental evidence showing that tau derived from human FTDP-17 disease may have distinct properties and unique seeding patterns. This underscores the importance of developing and using animal models with different tau mutations to fully study tauopathies leading to neurodegeneration.
A third paper, on which Dr. He served as first author, describes efforts he led to develop a new animal model incorporating human AD-associated tau strains to more closely resemble disease state in humans. Using this model, Dr. He injected tau strains obtained from people with different tauopathies and showed that specific tau strains recruit host proteins to take on the same conformation, and further, that each strain initiates unique, specific pathological immune responses in host immune cells (microglia communities). This, too, substantiates the idea that tau can be a “horse of many colors” when it comes to neurodegenerative disease spread. This impactful finding suggests that specific cell-derived factors in the host environment can help dictate tau spread and once identified, can serve as novel targets of intervention.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
Every Donation is a Step Forward in the Fight Against Alzheimer’s
Your donation powers cutting-edge research and helps scientists explore new treatments. Help bring us closer to a cure and provide valuable information to the public.
Donate Today