Alzheimer’s Disease Drug Shows Positive Clinical Trial Results
Findings are the first to show a statistically significant decrease in cognitive decline by an anti-amyloid drug.
Written By: BrightFocus Editorial Staff



Findings are the first to show a statistically significant decrease in cognitive decline by an anti-amyloid drug.
Written By: BrightFocus Editorial Staff
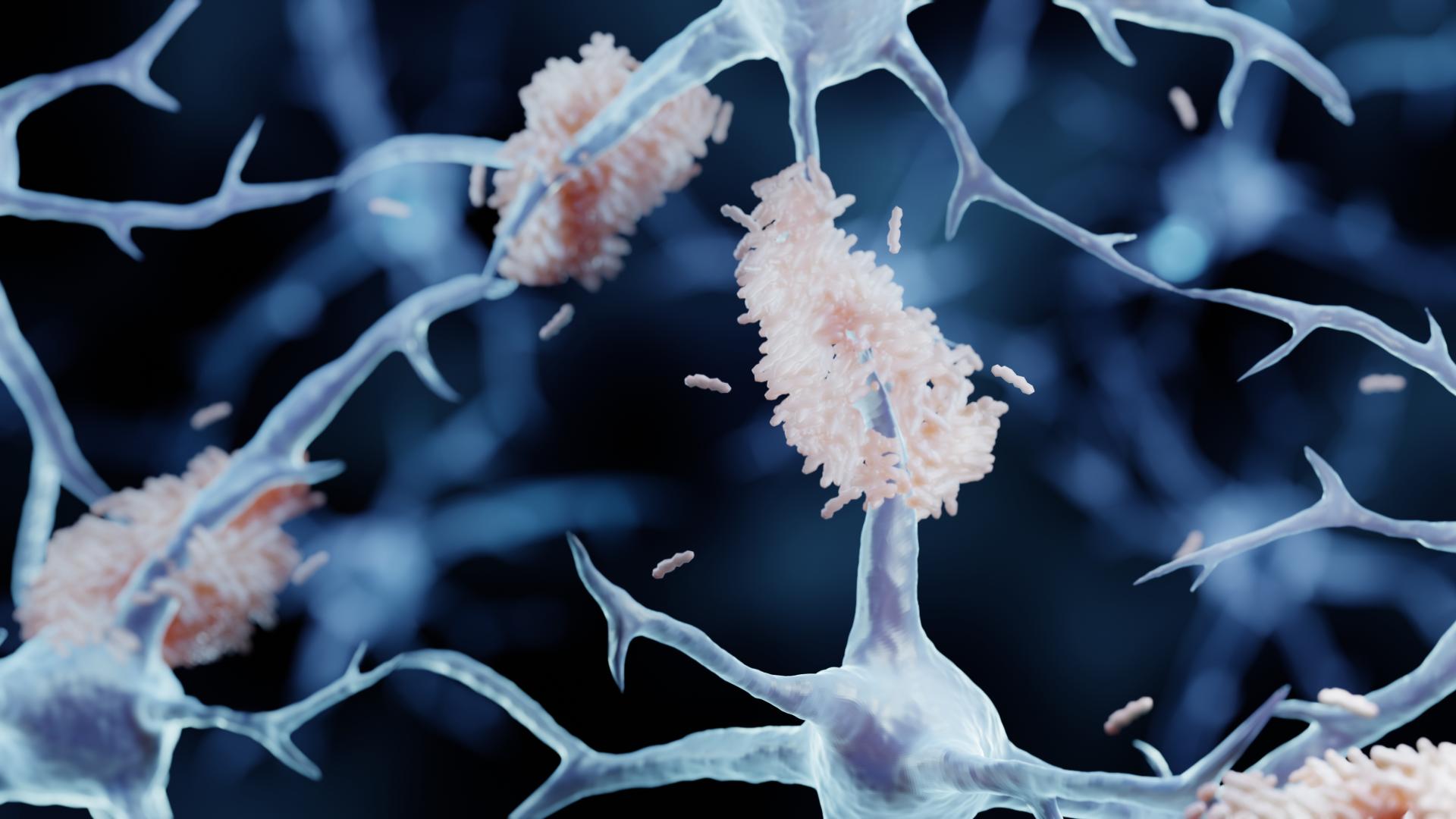
(Sept. 28, 2022) Clarksburg, MD—BrightFocus Foundation—a leading nonprofit funder of Alzheimer’s disease research for nearly 50 years—is encouraged by the positive topline results issued yesterday on the global Phase 3 clinical trial of Alzheimer’s drug lecanemab, which was reported to slow cognitive decline among people with Alzheimer’s disease.
The findings are the first to show a statistically significant decrease in cognitive issues by targeting amyloid, a protein expressed in many different organ systems. Eisai and Biogen reported lecanemab reduced cognitive decline by 27% through the removal of aggregated amyloid-beta (Aβ ) in the brains of people who received it (nearly 2,000 participants) compared to a placebo.
When amyloid gets misprocessed and accumulates, it causes neurodegeneration. A healthy brain can break down amyloid-beta and eliminate it, but in Alzheimer’s disease, Aβ forms insoluble plaques that are toxic to neurons and sometimes associated with memory loss and other changes.
Stacy Pagos Haller, President and CEO, BrightFocus Foundation, stated, “BrightFocus Foundation is excited for a potential new treatment that could slow the progression of Alzheimer’s disease and improve quality of life for the millions of people it devastates.” Haller added, “Our team of expert Alzheimer’s disease researchers are actively exploring multiple scientific pathways that have the most promise to end Alzheimer’s; we won’t stop until there’s a cure.”
Eisai will present the study results on Nov. 29 at the Clinical Trials on Alzheimer’s Congress (CTAD) and stated that findings will be published in a peer-reviewed medical journal. Eisai already applied for accelerated FDA approval of the drug based on a smaller, earlier-stage clinical trial and said they will now submit the new trial results to the FDA and expect a decision to be made by early next year.
Scientific experts from BrightFocus Foundation are available to discuss brain and vision research.
Diane Bovenkamp, PhD, vice president, Scientific Affairs; Sharyn Rossi, PhD, director of scientific programs, neuroscience; and Preeti Subramanian, PhD, director of scientific programs, vision science, at BrightFocus Foundation, are available for comments.
To arrange a media interview, please contact:
Kaci Baez, BrightFocus Foundation
kbaez@brightfocus.org
(301) 556-9370
Julia Roth, BrightFocus Foundation
jroth@brightfocus.org
(301) 556-9382
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Through its flagship research programs — Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research— the Foundation has awarded nearly $300 million in groundbreaking research funding over the past 51 years and shares the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
