Treatments for Macular Degeneration
All macular degeneration starts as the dry form, where the macula increasingly thins through early, intermediate, and advanced stages. Dry age-related macular degeneration (AMD) can progress to the wet, more advanced, form, in which tiny, fragile blood vessels in the eye can leak and trigger inflammation. These two types of AMD have different treatment plans. Continue reading for more details.

Together, We Can Stop Macular Degeneration
As many as 20 million Americans have macular degeneration. Your support helps fund research studies to defeat this disease and provide vital information to the public.
Treating Macular Degeneration
If you’ve been diagnosed with AMD, it’s important to see your eye doctor regularly to track how quickly your disease progresses. Your doctor can tell you how to control risk factors for the disease and show you how to use the Amsler grid, a simple visual test so that you can detect subtle changes in your vision.
At the first sign of any visual changes, no matter how small they seem, you should make an appointment with your eye care provider.
Dry AMD Treatments
People with intermediate-stage dry AMD may benefit from taking a special mix of supplements to decrease their risk of losing central vision. In clinical trials, an over-the-counter combination of vitamins and minerals called the AREDS2 formula showed benefit in preventing the progression of intermediate dry AMD to late dry AMD. AREDS2 also may slow the development of wet AMD, the less common form of the disease.
AREDS2 Vitamin Supplement Formula
It’s important to find the right vitamins since many are marketed for eye health, but only a few have formulas that have proven effective. The recommended formula is:
- 500 mg of vitamin C
- 400 IUs of vitamin E
- 10 mg of lutein
- 2 mg of zeaxanthin
- 80 mg of zinc
- 2 mg of copper
You should always speak with your doctor before adding medications to your regimen.
FDA Approved Treatments
Two U.S. Food and Drug Administration-approved treatments for dry AMD are intended for people in a late stage of the disease who have been diagnosed with geographic atrophy. The first, Syfovre, was approved in February 2023, followed by Izervay in September 2023. Both are injections shown to reduce the rate of geographic atrophy lesion growth.
In 2024, the Valeda® Light Delivery System was approved for use in dry AMD. Valeda uses multiple wavelengths of light (red and near-infrared) to stimulate cellular processes within the retina, including mitochondrial activity, which can help reduce inflammation and promote healthy cell function.
Wet AMD Treatments
Wet AMD can be treated with injections of angiogenesis inhibitors into the eye, with photodynamic therapy, or with laser surgery. None of these treatments will cure wet macular degeneration, but each may slow the rate of vision decline or stop further vision loss.
Anti-VEGF and Other Injected Treatments
Anti-VEGF shots block vascular endothelial growth factor, a key molecule in the production of new blood vessels in a process called angiogenesis, and are injected into the back of the eye, which has been numbed beforehand.
Photodynamic Therapy
Photodynamic therapy (PDT) is most effective in a subtype of wet macular degeneration called predominantly classic subfoveal AMD, in which blood vessel growth and leakage in the fovea are well defined. PDT is rarely used now that there are drugs that specifically block the vessel-promoting VEGF protein.
Laser Surgery
Laser photocoagulation surgery was the first treatment used for wet macular degeneration, but it is only an option for a small number of patients.
Side Effects
For a complete list of side effects associated these treatments, please refer to our comprehensive list of Wet and Dry AMD treatments.
Frequently Asked Questions
Search for a Macular Degeneration Clinical Trial
Clinical trials are crucial to advancing the most effective medical approaches. Today’s studies will lead to new standards of care in the future.

Resources
Recent Resources & Information
Expert Information
Gene Therapy Advances in Age-Related Macular Degeneration
Gene therapy treatments may one day free people with age-related macular degeneration (AMD) from the need for frequent eye injections.

Expert Information
How Retinal and Brain Implants Restore Partial Sight
Artificial vision systems utilizing these technologies show promise for individuals with profound vision loss in early trials.

Expert Information
Emerging Treatments Offer New Hope for Dry and Wet Age-Related Macular Degeneration
Promising new treatments in late-stage clinical trials could transform how we treat age-related macular degeneration.
Downloadable Resource
Treatments for Macular Degeneration
Find information on treatment options for dry and wet age-related macular degeneration.
Expert Information
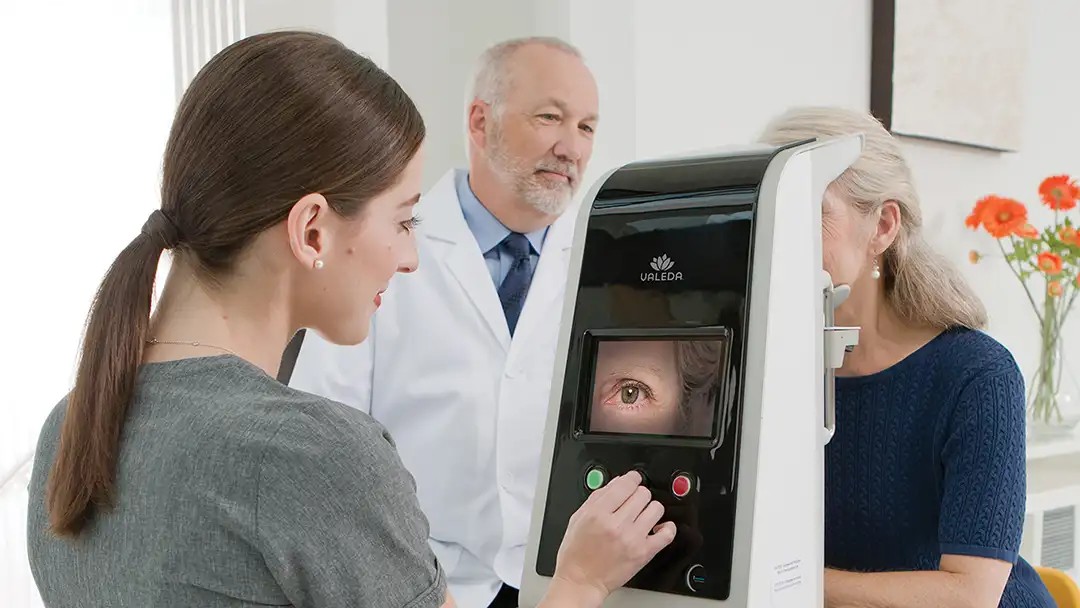
What to Know About Light Therapy for Dry Macular Degeneration
A new treatment using red light may help protect vision for people with dry age-related macular degeneration. Here’s what a retina specialist wants you to know.

Macular Chats
Light Therapy and Macular Degeneration: What You Need to Know
Join us for a conversation about light therapy and macular degeneration, where we’ll explore how different types of light—blue, red, and sunlight—affect the retina, the science behind light therapy, and the newly approved Valeda red light therapy.


