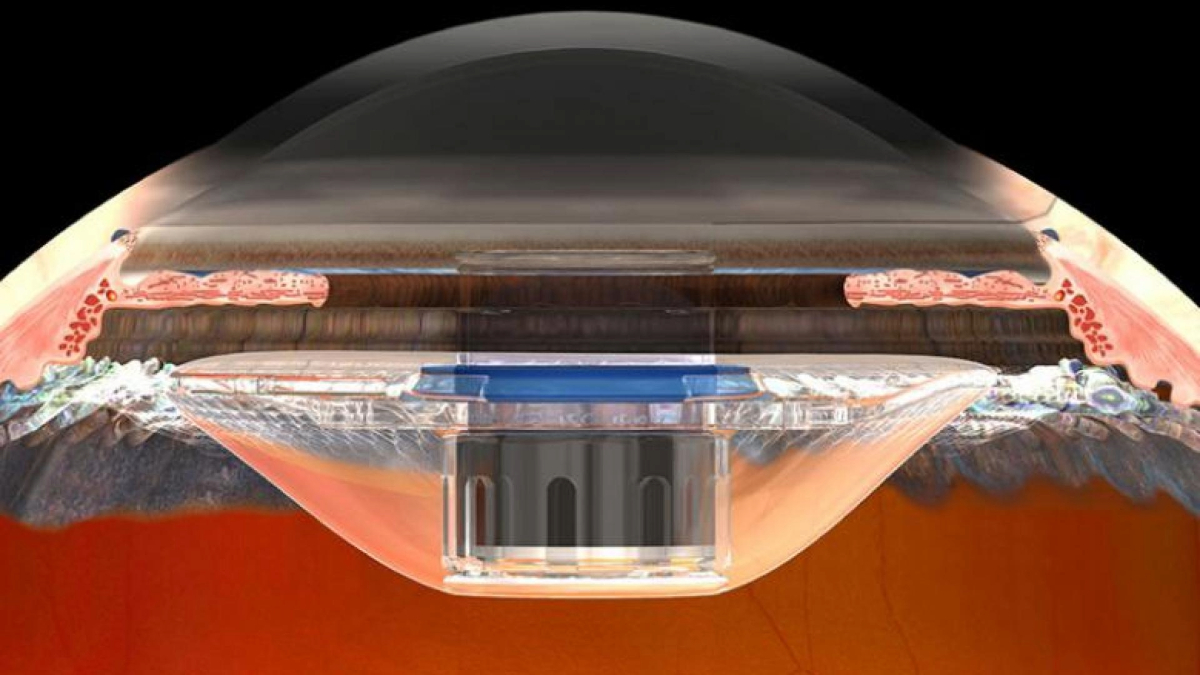
Some patients with age-related macular degeneration (AMD) lose their central vision in both eyes. This makes it difficult to read, recognize faces, or watch television. Fortunately, the more peripheral retinal beyond the centrally-located macula is not usually affected. The implantable miniature telescope (IMT) takes advantage of this fact by enlarging objects in the center of the visual field so they can be seen by the intact parts of the retina around the macula.
The IMT has been tested in clinical trials and is approved by the FDA for patients with corrected vision of 20/160 – 20/800) that is caused by bilateral end-stage AMD. Patients with active wet AMD are not eligible. Patients must also achieve at least a 5-letter improvement on the visual acuity chart using a trial external telescope. This trial is an important predictor of the benefit from an IMT and can be used to simulate vision after telescope implantation. They must also have their natural lens in place at the time of IMT implantation; that is, no prior cataract surgery in that eye.
In January of 2017, VisionCare Ophthalmic Technologies™ announced approval by the FDA to initiate a new clinical trial to investigate the safety and efficacy of the telescopic implant in post-cataract surgery patients.
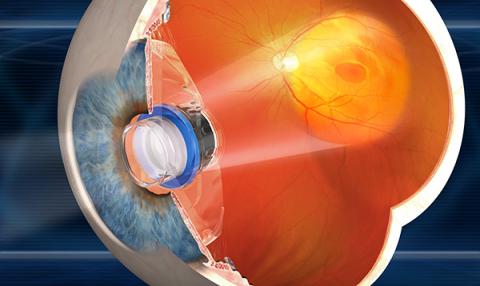
The Surgical Procedure
The IMT is implanted immediately after removal of the natural lens. Removal of the natural lens and implantation of a plastic lens is routine in standard cataract surgery. Implantation of an IMT uses related techniques, and an IMT is implanted instead of the standard plastic intraocular lens (IOL). The IMT is about the size of a pea, and sits in the eye where the natural lens used to reside. Since the IMT is larger than an IOL, the incision required to insert it is larger. Complications of the surgery are infrequent, and include inflammation. An approximately 25% reduction in the number of cells lining the inside of the cornea commonly occurs, so the surgery is riskier for patients who start out with low numbers of corneal endothelial cells. These cells are needed to keep the cornea clear.
Two IMT Models
Two models of IMT are available: one with 2.2-times magnification and the other with 2.7-times magnification. This higher magnification version provides better visual acuity (by about a line on the eye chart), but slightly smaller visual field (20 versus 24 degrees). In the clinical trial, patients with the higher magnification telescope gained, on average, 3.6 lines on the eye chart, which is similar to improving from 20/160 to 20/80, or 20/200 to 20/100. According to a questionnaire, this resulted in a significant improvement in quality of life.
Who Benefits the Most?
IMTs cause a decrease in depth perception, but this can improve with learning and experience over time. It is important to work with a low vision specialist and occupational therapist before and after surgery to determine whether the surgery is suitable for each person and to teach techniques for using the device. Generally, patients who are motivated to use their remaining vision benefit the most. These are patients who are already using low vision aids to sign their own checks and read large print. After implantation of an IMT, patients must practice looking at moving objects while sitting still, or looking at stationary objects while walking. It takes time and patience to get used to seeing magnified objects in the IMT-implanted eye, which now has tunnel vision, and using the other eye for peripheral vision. This has been compared to using monovision contact lenses; one to see distance and one for near.
The best results will be obtained from centers where surgeons have experience with the specialized implantation procedure. These centers must also have affiliated low vision optometrists and occupational therapists, both of whom are experienced with IMT training and are available both pre- and post-operatively.
About BrightFocus Foundation
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
- Clinical Trials