Learn what gene therapy is and the potential strategies to use these methods to treat glaucoma.
Introduction
Glaucoma is a leading cause of irreversible vision loss due to progressive degeneration of the optic nerve, which transmits visual information from the eye to the brain. While several genetic and environmental risk factors contribute to the development and progression of the disease, the only proven method to treat glaucoma and slow vision loss consists of lowering eye pressure with eye drop medications or surgeries. Unfortunately, glaucoma tends to progress despite these conventional therapies. As we gain a better understanding of glaucoma disease mechanisms, such as by identifying underlying genetic defects, more effective therapies can be developed. These novel treatments, including gene therapies, show much promise in laboratory settings and hopefully can proceed to clinical trials in the next few years.
What Is Gene Therapy?
With ocular gene therapy, genetic material such as DNA or RNA is injected into the eye to achieve a therapeutic effect. One common and effective way to administer gene therapy is by using benign virus particles, or vectors, that carry the genetic material into the cells of interest. Alternatively, cells can be harvested or developed from stem cells, treated with gene therapy in a dish in the laboratory, and then injected into the eye. The advantages of gene therapy include the ability to specifically target known disease mechanisms and cells and the potential that a one-time treatment will result in a long-term therapeutic effect and possibly a cure. By injecting the gene therapy vector directly into the eye, the treatment reaches the desired target more effectively than by eye drops or oral medications. The eye is well suited for gene therapy because it forms a separate compartment where inflammation is relatively muted compared to other organs; this is referred to as “immune privilege.”
Gene therapy can be used to address genetic risk factors by either replacing or silencing defective genes that contribute to the development and progression of glaucoma. Gene therapy can also be applied to enable cells within the eye to produce drugs continuously, thereby replacing the need for daily eye drop medication.
Several ocular gene therapies have already been developed in the laboratory and moved into clinical trials for human patients, mostly for the treatment of blinding retinal diseases. Generally, these eye injections are well established and tolerated. In December 2017, the U.S. Food and Drug Administration (FDA) approved the first viral ocular gene therapy, Luxturna™, by Spark Therapeutics, Inc., for the treatment of one form of retinal childhood blindness.
Potential Gene Therapy Strategies for Glaucoma
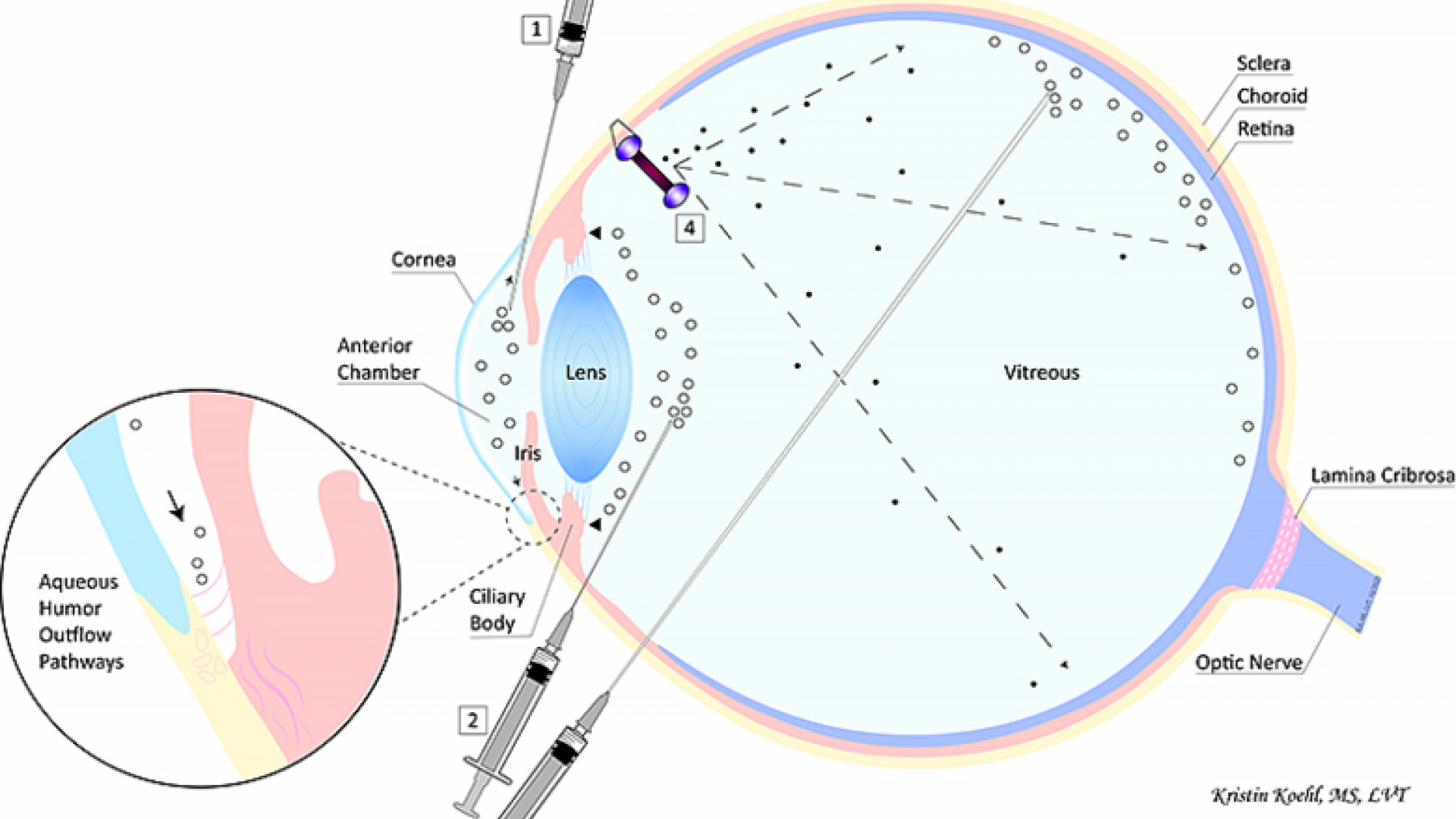
The numbers listed in the paragraphs below reference the illustration at the top of the page.
Some of the most promising gene therapy strategies being developed for the treatment of glaucoma include the reduction of eye pressure by either increasing fluid drainage (1) or decreasing fluid production (2). These are the same strategies currently pursued by routine medical and surgical treatments, but gene therapies have the potential to achieve a long-term therapeutic effect following a one-time injection. Increased fluid drainage via aqueous humor outflow pathways can be achieved by injecting the gene therapy vector into the anterior chamber (1). Decreased fluid production occurs by targeting the faucet within the eye, the ciliary body, via injection into the vitreous (2).
There is a general understanding among glaucoma experts that effective glaucoma therapy consists not only of eye pressure reduction but also the direct treatment and protection of neural cells in the retina, most importantly retinal ganglion cells, and their extensions, or axons, that connect via the optic nerve to the brain. This treatment strategy is known as “neuroprotection.” The retina and optic nerve can be directly targeted by gene therapy viral vector injection into the vitreous (3). Alternatively, cells can be treated in a dish in the laboratory to enable them to produce drugs with neuroprotective effects. These cells can then be administered into the vitreous directly by injection (3) or enclosed in a capsule that is implanted into the eye (4). The latter approach is currently being studied in a clinical trial for glaucoma: Genetically engineered cells are contained in the NT-501 capsule developed by Neurotech Pharmaceuticals, Inc.; these encapsulated cells continuously release ciliary neurotrophic factor (CNTF), a protein that has been shown to be neuroprotective in glaucoma animal models.
Summary
Much progress has been made in recent years in the development and clinical testing of ocular gene therapies. Promising gene therapies have been developed in the laboratory for the comprehensive treatment of glaucoma by reducing eye pressure and providing neuroprotection of the retina and optic nerve. As we gain more understanding of glaucoma disease mechanisms, we will be able to target them specifically using gene therapy to achieve safe, effective, and long-term prevention of vision loss.
About BrightFocus Foundation
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Through its flagship research programs — Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research— the Foundation has awarded nearly $300 million in groundbreaking research funding over the past 51 years and shares the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
- Genetics
- Treatments