Updated on January 29, 2025
Glaucoma is a group of eye diseases with different symptoms, but the common feature among all types of glaucoma is optic nerve damage. Much like Alzheimer’s disease is a neurodegenerative disease of the brain, glaucoma is considered a neurodegenerative disorder of the optic nerve.
In glaucoma, optic nerve cells degenerate and eventually lead to cell death, which can cause permanent vision loss. This can occur because of elevated eye pressure, poor blood flow, and many other possible factors.
Learn more about how scientists around the world are studying ways to regenerate the optic nerve.
What is the Optic Nerve?
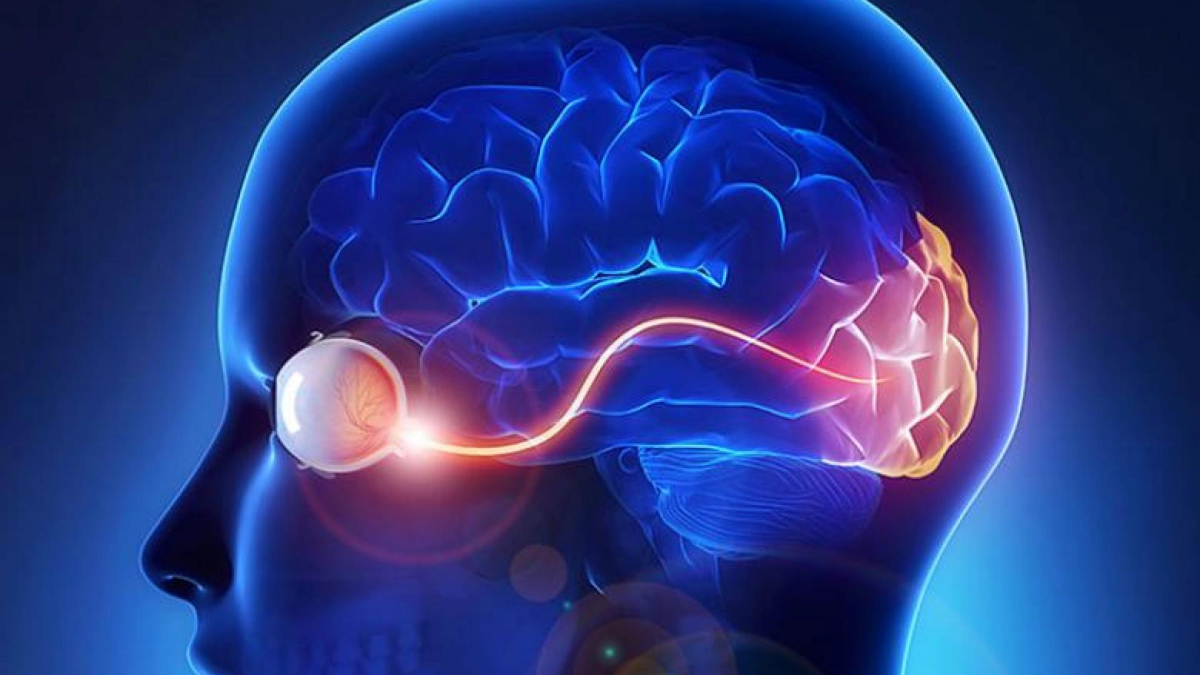
The optic nerve is composed of approximately 1.5 million nerve fibers at the back of the eye that carry visual messages from the retina to the brain. When light hits the retina, the photoreceptors (light-sensitive cells) receive and transmit this information to other specialized cells, including the final cell type in the chain, called the retinal ganglion cells. These cells reside in the retina, but their output “cables” or “fibers,” called axons, extend from the optic nerve to specific regions in the brain. Retinal ganglion cells play a critical role as the output nerve cell of the eye that transmits visual information to the brain. In the brain, the visual information is further processed to give us sight.
During an eye exam, your eye doctor can visualize the optic nerve with the help of special lenses. The axons of the optic nerve are bundled and located in the back of the eye. This “optic disc” is seen in the back of the eye along with blood vessels. In optic nerve degeneration related to glaucoma, the optic disc displays changes that are characteristic of glaucoma, which your doctor may refer to as “cupping.”
Video Showing the Optic Nerve
“Cupping”
The normal optic nerve has a healthy appearing “rim” of tissue, which is assessed by both the contour of the rim as well as the color. “Cupping” is the result of changes in the optic nerve related to optic nerve degeneration, where there is a backward bowing of the central part of the disc. When your optic disc is seen in three dimensions, the “cupping” can be very obvious to your eye doctor. In the video below, the section beginning at approximately 29 seconds demonstrates the cupping effect.
Other physical features of the optic disc can suggest glaucoma, such as thinning of the “rim,” focal notches or loss of rim tissue, and bleeding (also called disc hemorrhages). The thinning of the rim that characteristically first affects regions of the optic disc in glaucoma is related to the typical visual field deficits seen in glaucoma patients.
When “cupping” related to glaucoma is very severe (when it affects most of the rim of the disc such that very little healthy tissue remains, for example), loss of central vision and ensuing blindness can result, although this typically occurs in the very late stages of glaucoma.
Video Demonstrating the “Cupping” Effect
An excellent visualization of optic nerve “cupping” starts at approximately 29 seconds into the video.
What Happens to the Optic Nerve in Glaucoma?
In glaucoma, the axons of these optic nerve cells degenerate, and eventually lead to cell death. There are many different factors that contribute to the process. One major risk factor is eye pressure. However, it is possible to have elevated eye pressure, or ocular hypertension, and show no changes of optic nerve degeneration (although one would need to be monitored over time for signs of degeneration). Conversely, it is possible to have normal eye pressure and significant optic nerve degeneration, which is often called “normal tension glaucoma” or “low tension glaucoma.”
What we do know is that there is likely mechanical damage at the site where the optic nerve inserts into the back of the eye. This is because the axons of the optic nerve leave the eye by passing through a structure called the lamina cribrosa, a mesh-like structure composed of pores or holes through which the axon bundles must cross. The site of initial damage may be the axons as they pass through the lamina cribrosa as the optic nerve exits the eye.
Another contributing factor may be blood flow to the optic nerve. Some researchers believe that in cases of normal-tension glaucoma, impaired blood flow to the optic nerve may play a more important role in the degenerative process.
Unfortunately, like other central nervous system regions such as the spinal cord, the regenerative capacity of the optic nerve is limited. There are many reasons for this, including the fact that retinal ganglion cells cannot repair or regenerate themselves after injury without significant help.
The environment in which the optic nerve resides also contains signals that inhibit regeneration and lacks signals to stimulate regrowth. Therefore, glaucoma is currently an incurable disease. However, scientists around the world are studying ways to regenerate the optic nerve, not only to help glaucoma patients, but also because the optic nerve is particularly helpful in studying central nervous system regeneration.
Can the Optic Nerve Regenerate?
Regeneration of the optic nerve to restore vision requires several steps:
- The damaged retinal ganglion cells need to stay alive and not die.
- Surviving brain cells (neurons), which normally do not grow in adults, need to go “backwards in time” and become more “immature” in order to regrow, as they had done during early development.
- Nerve fibers that re-grow need to overcome signals that inhibit growth.
- Regenerating axons (nerve fibers) have to connect to the appropriate location in the visual targets of the brain.
For the first step, researchers have made important progress in understanding the factors that help retinal ganglion cells survive and prevent degeneration. In a U.S.-based clinical trial, ciliary neurotrophic factor-secreting implants have been assessed for safety, vision preservation, and visual improvement in patients with severe glaucoma.
This trial is exciting in its potential to validate a completely new category of treatments for glaucoma patients. Currently, treatment is limited to medications or surgery that lower intraocular pressure, which is important, but not sufficient as there are some patients whose glaucoma continues to get worse even with low eye pressure.
For the remaining steps, scientists are studying ways in which to instruct injured neurons to sprout axons (fibers) and regenerate by supplying factors that stimulate and instruct the process. Scientists are also exploring axonal guidance, which provides cues for the axons to grow in the correct direction and to the proper location.
There is even some recent evidence in animal models of glaucoma that manipulating specific genes and stimulating retinal activity may lead to axon regeneration and reconnections with the correct targets in the brain.
Researchers now believe that in order to achieve meaningful optic nerve regeneration, multiple treatments that stimulate growth and suppress the tissue’s growth inhibition signals need to be combined. It is a tall order but one that is being actively investigated around the world, including by several BrightFocus-funded researchers.
Protecting the Optic Nerve (Neuroprotection)
Current treatments for the degenerative process of glaucoma involve lowering eye pressure. This is achieved using eye drops, laser surgery, or incisional eye surgery.
While lowering eye pressure has been proven to slow visual field loss, the unfortunate reality is that some patients whose pressures have been lowered still continue to show signs of worsening. Therefore, researchers are working to develop novel treatments that slow or halt optic nerve degeneration, also called neuroprotection.
One of the currently available treatments that may have neuroprotective effects is the eye drop Alphagan (brimonidine), which is used to lower eye pressure in glaucoma patients.
A recent randomized control trial compared patients with normal pressure glaucoma who received either timolol (another eye drop commonly used to treat glaucoma) or brimonidine. Despite the fact that both groups had similar eye pressure lowering, patients receiving brimonidine had visual fields that did not deteriorate as much as the visual fields of patients receiving timolol.
However, there are a few caveats in interpreting this study, including the fact that more patients dropped out of the study in the brimonidine group as compared to the timolol group.
In addition to eye drops, animal studies have shown that some commonly used systemic medications that patients already take, such as insulin and GLP-1 receptor agonists (for example, Ozempic), are neuroprotective. In the case of insulin, animal studies have shown that it helps retinal ganglion cells that comprise the optic nerve to regrow their dendrites. Treatment using GLP-1 receptor agonists has been shown to be neuroprotective in animal studies.
More clinical studies are needed on neuroprotective treatments that demonstrate that vision is truly preserved, retinal ganglion cells survive, and optic nerve degeneration is halted.
BrightFocus Foundation supports scientists working diligently on optic nerve regeneration to restore vision. Check out National Glaucoma Research grant recipients who are advancing our understanding of optic nerve regeneration below.
About BrightFocus Foundation
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Through its flagship research programs — Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research— the Foundation has awarded nearly $300 million in groundbreaking research funding over the past 51 years and shares the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
- Disease Biology
- Vision Restoration
Help Fund More Research Breakthroughs.