Explore key milestones and see how our researchers are improving lives.
1973
Our story starts here.
BrightFocus was founded on May 15, 1973. Our charter was to fund research to find cures for the most pressing health problems.

The BrightFocus national office in Clarksburg, Maryland.
1979
National Glaucoma Research, a program of BrightFocus Foundation, begins.
1982
Surgeons at the University of Utah transplant the first permanent artificial heart into a patient.
BrightFocus funded early research supporting the artificial heart, one of the long-sought holy grails of modern medicine, and provided critical early funding to Dr. Willem Kolff, a Dutch physician known as the father of artificial organs. Dr. Kolff led the team that worked on the artificial heart that was implanted into 61-year-old Barney Clark in 1982 and designed the first artificial kidney.

Dr. Willem Kolff, courtesy of the Spencer S. Eccles Health Sciences Library, University of Utah.
1985
Alzheimer’s Disease Research, a program of BrightFocus Foundation, begins.
1992
Researchers uncover new genetic causes of inherited Alzheimer’s disease.
Scientists including Alzheimer’s Disease Research grantees Drs. Rudolph Tanzi, Gerard Schellenberg, John Hardy, and Peter St. George-Hyslop discover a new area of chromosome 14 associated with familial Alzheimer’s disease, setting the stage for an improved understanding of hereditary genes for Alzheimer’s.

Dr. Gerard Schellenberg (left) and colleagues at the University of Washington.
1993
Key gene identified as a risk factor for Alzheimer’s.
Alzheimer’s Disease Research-funded scientists Drs. Allen Roses, Peter St. George-Hyslop, Thomas Bird, and Gerard Schellenberg identify apolipoprotein E (APOE) as a major risk factor for late-onset Alzheimer’s disease. Today, APOE is the most common gene associated with Alzheimer’s.
1993-1995
Alzheimer’s research expands in Europe.
BrightFocus helped established Alzheimer’s research programs in the Netherlands (1993), Germany (1995), and Belgium (1995), broadening philanthropic support for Alzheimer’s research internationally.

BrightFocus-supported Alzheimer’s research in Europe begins in the Netherlands in 1993.
1995
Researchers lay the groundwork for a better understanding of age-related macular degeneration—a disease that leads to loss of central vision.
Renowned English ophthalmologist Alan C. Bird, a member of the BrightFocus Scientific Review Committee, and his team, coin the terms “early and late age-related macular degeneration” and “dry and wet late age-related macular degeneration,” creating an international classification and grading system for age-related maculopathy and age-related macular degeneration.
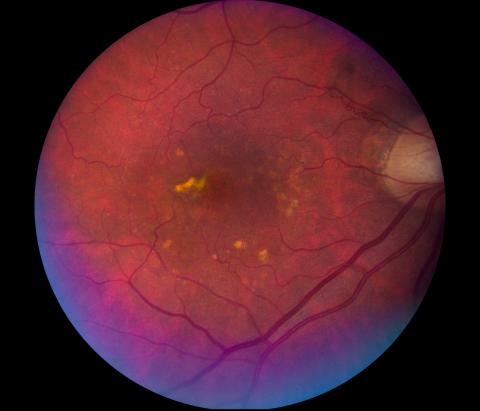
Image of a retina with dry age-related macular degeneration.
1995
Researchers lay the groundwork for a better understanding of age-related macular degeneration—a disease that leads to loss of central vision.
Alan C. Bird, MD
Emeritus Professor and Consultant at the Institute of Ophthalmology at the Moorfields Eye Hospital, London
Best known for his work on retinitis pigmentosa and research into inherited retinal degeneration, Dr. Bird studied neurology and neurosurgery, but later turned to ophthalmology. While at the Institute of Ophthalmology, he worked with numerous fellows in a variety of multidisciplinary activities involving electrophysiology, specialized imaging, psychophysics, immunology, and pathology—which resulted in the development of new technologies to define the clinical characteristics of retinal disease. His studies have also correlated abnormal gene expression with metabolic dysfunction at the cellular level, which has led to a clearer understanding of retinal degenerative diseases, and has had significant implications for clinical management of these disorders, including better genetic counseling for patients and the development of new treatment approaches, including gene therapy. He has received many awards, including the Duke Elder, the Doyne and Bowman medals, the Prix Chauvin, and the Helen Keller Prize for Vision Research. Dr. Bird has undertaken extensive international work in Africa, tackling river blindness, and in Jamaica, examining the retinal changes that occur in patients with sickle cell disease.
1997
Stanley Prusiner, MD, receives Nobel Prize for groundbreaking prion discovery with links to Alzheimer’s disease.
Grantee Stanley Prusiner, MD, was awarded the 1997 Nobel Prize in Physiology or Medicine for his groundbreaking discovery and definition of prions, infectious proteins in the brain that cause mad cow disease in cattle and Creutzfeldt-Jakob disease in humans. There are similarities between the loss of brain function in prion diseases and in Alzheimer’s disease, and an understanding of how prion diseases begin and develop may one day lead to a treatment and a cure for Alzheimer’s. Dr. Prusiner served as co-chair of our Alzheimer’s Disease Research Scientific Review Committee and became an honorary BrightFocus board member in 2007. In 2010, President Obama awarded him the National Medal of Science, the country’s highest honor for science and technology.

Dr. Stanley Prusiner
1997
Stanley Prusiner, MD, receives Nobel Prize for groundbreaking prion discovery with links to Alzheimer’s disease.
Dr. Prusiner Accepts the BrightFocus Award for Scientific Impact
1999
Macular Degeneration Research, a program of BrightFocus, begins.
2000
Paul Greengard, PhD, receives Nobel Prize for his foundational insight that nerve cells communicate with each other.
A 1986 Alzheimer’s Disease Research grant led Paul Greengard, PhD, to initiate a series of milestone experiments into protein phosphorylation in specific brain regions related to Alzheimer’s disease. His most famous discovery, for which he was awarded the Nobel Prize in Physics or Medicine in 2000, is that nerve cells communicate with each other through chemical and electrical signaling working in tandem, known as signal transduction. This discovery laid the foundations of modern neuroscience.

Dr. Paul Greengard
2003
Researchers develop first optical test to diagnose Alzheimer’s disease.
Following his exciting discovery that Alzheimer’s disease can be detected in the lens of the eye, Lee Goldstein, MD, PhD, and his team developed new optical tests that can potentially diagnose and monitor the disease from the beginning stages, a discovery that could lead to an accessible, noninvasive way to detect, track, and treat Alzheimer’s.

Dr. Lee Goldstein (far right) and his team.
2005
BrightFocus expands Alzheimer’s research in Europe through the establishment of Alzheimer’s France.
Alzheimer’s research in Europe expands to France.
2005
First anti-VEGF treatment for wet age-related macular degeneration.
Macular Degeneration Research grantee Peter Campochiaro, MD, is among the first to prove the benefits of suppressing vascular endothelial growth factor (VEGF), leading to the development of a novel anti-VEGF age-related macular degeneration treatment method that’s still the gold standard for helping to slow or stop further vision loss in wet age-related macular degeneration patients.
2006
Researchers discover the “complement pathway” in age-related macular degeneration—central to the development of the first and only treatment for geographic atrophy, a leading cause of blindness.
Research funded by Macular Degeneration Research helped lay the foundation for the discovery and understanding of the complement pathway, part of the immune system, and its involvement in onset of age-related macular degeneration.

2008
Launch of educational workshop for early-career scientists.
BrightFocus offers its first workshop dedicated to Alzheimer’s disease research, now known as Alzheimer’s Fast Track, for PhD students and postdoctoral fellows to learn from world-renowned experts in the field. Today, the program includes Macular Fast Track and Glaucoma Fast Track and has educated thousands of early-career scientists from around the world.

Alzheimer’s Fast Track students receive feedback on their mock grant proposal from Laura Cox, PhD, assistant professor in the department of neurology at the Ann Romney Center for Neurologic Diseases at Harvard Medical School and Brigham and Women’s Hospital, one of the program’s speakers.
2008
Launch of educational workshop for early-career scientists.
2010
Grantees create the first noninvasive technique to measure eye pressure.
Grantee Joel Schuman, MD, and his team create the first noninvasive technique to measure eye pressure—the most important factor to consider in glaucoma. For the first time, scientists can visualize and measure how fast drainage in the front of the eye occurs without touching the eye and without any bright lights.
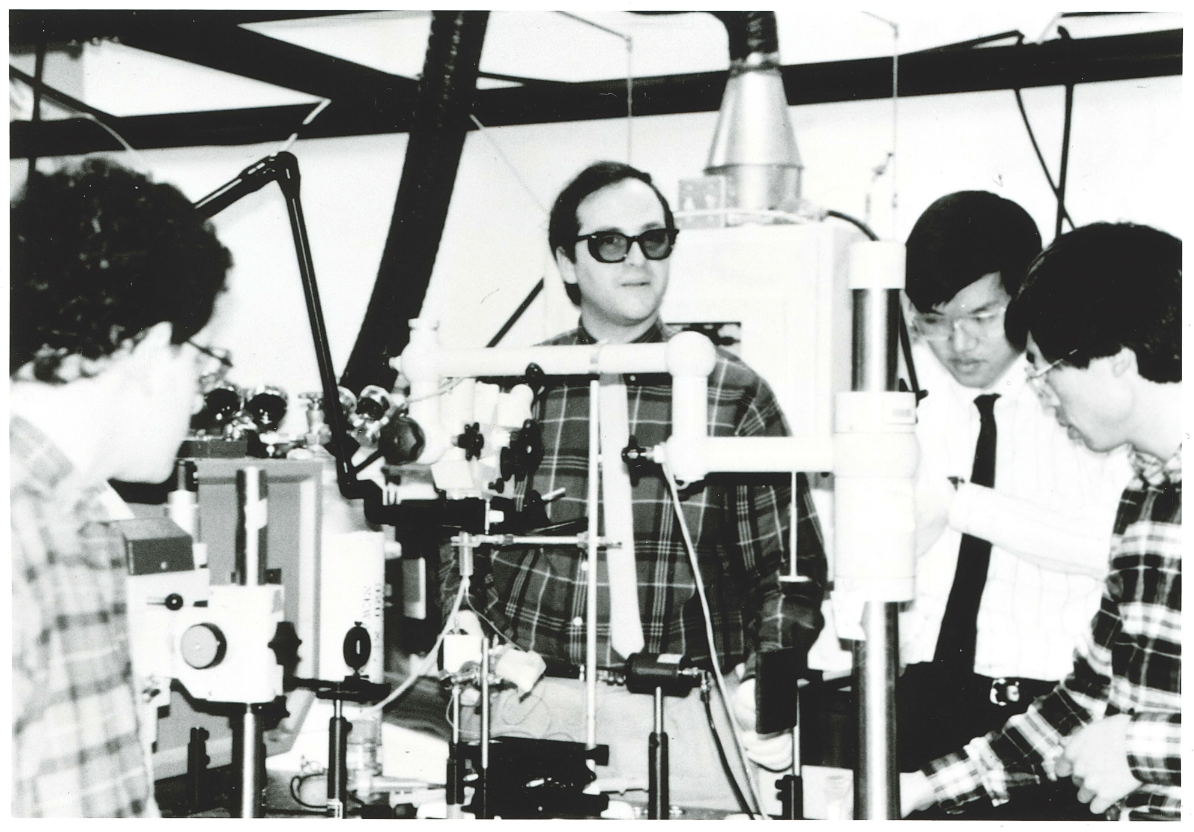
Dr. Joel Schuman (center) and his team circa 1995.
2010
Grantees create the first noninvasive technique to measure eye pressure.
Breaking Ground in Glaucoma: Spotlight on Dr. Joel Schuman
In 1995, with his ophthalmology residency a few years behind him, Joel S. Schuman, MD, received a BrightFocus grant to delve into the genetics of glaucoma. And before a decade passed, he and his colleagues were the first to identify a molecular marker for human glaucoma.
With several BrightFocus projects, Schuman has led research to develop advanced imaging for glaucoma and other vision diseases, which has led to advances in detection and early treatment.
Most notably, Schuman was a member of the team that developed a groundbreaking medical imaging procedure called optical coherence tomography (OCT) that creates a 3D map of the eye. This quick and noninvasive procedure allows ophthalmologists to measure the thickness of the optic nerve and to assess the nerve and surrounding tissue for signs of stress caused by pressure elevation. OCT is rapidly becoming the practice standard for diagnosing glaucoma and monitoring its progression. It makes it possible to intervene and reach treatment decisions earlier and is invaluable in helping to preserve precious sight.
“I cannot tell you how many times I’ve heard, ‘If it weren’t for BrightFocus, I could never do this research,” said Dr. Schuman. “What’s especially valuable about the BrightFocus mission is its focus on young investigators,” he continued. “They are our future, and BrightFocus has the foresight to invest in them, as the organization invested in me.”
“I have been privileged to be involved in research that has made a difference in people’s lives around the world. This would not have been possible without BrightFocus.”—Dr. Joel Schuman
2013
Expanding research into sex-based differences in Alzheimer’s disease.
Three BrightFocus-funded investigators win an award to investigate gender-based differences in Alzheimer’s disease diagnosis and treatment. Their work examines whether certain genes are expressed differently in men and women to explain the increased risks for Alzheimer’s in women and explores expanding technology to better predict sex-based differences in Alzheimer’s disease.
BrightFocus researchers explore the role of gender in Alzheimer’s.
2014
A new inflammatory marker discovered for wet age-related macular degeneration.
Macular Degeneration Research grantees Sarah Doyle, PhD, Matthew Campbell, PhD, and others in Dublin, Ireland, discover that the inflammatory marker IL-18 could be used to prevent wet AMD and lead to a better understanding of how the immune system could be managed to reduce vision loss.

Sarah Doyle, PhD, and Matthew Campbell, PhD
2014
A new inflammatory marker discovered for wet age-related macular degeneration.
2015
Closer to the possibility of a vaccine to help rid the brain of toxic tau.
A team led by Alzheimer’s Disease Research grantees Kristen Funk, PhD, and Marc Diamond, PhD, brings us closer to the possibility of a vaccine by researching antibody immunotherapies to help rid the brain of toxic tau—and possibly keep it from spreading—in Alzheimer’s disease. Results of in vitro experiments show that monoclonal antibodies they’ve developed can disarm the harmful effects of abnormal tau protein, laying the groundwork for present-day clinical trials using anti-tau antibodies.

Kristen Funk, PhD, and Marc Diamond, PhD
2015
Bringing rigorous science to caregiving.
BrightFocus convenes the first national research summit on dementia caregiving, recognizing the evidence behind home-based dementia care as a critical component of care services for people with Alzheimer’s disease and related dementias in the coming decades.
A collaborative grant awarded in 2020 to Quincy Samus, PhD, would later advance the dissemination and translation of MIND at Home, an evidence‐based dementia care coordination model, into practice.

Dr. Quincy Samus
2015
Advances in gene-editing technology improve the study and treatment of age-related macular degeneration.
BrightFocus-funded researchers, including Don Zack, MD, PhD, expand CRISPR gene-editing technology and develop a modified CRISPR technique that improves the speed and efficiency of gene function studies, aids in the development of new cellular models of diseases, and eventually could help treat genetic conditions.

A 3D rendering of the treatment and adjustment of DNA.
2015
First clinical trial to test a new glaucoma treatment focused on neuroprotection and vision restoration.
Grantee Jeffrey Goldberg, MD, PhD, leads a BrightFocus-funded, first-of-its-kind Phase 2 clinical trial to test a new glaucoma treatment consisting of an experimental cell therapy to protect the optic nerve. In this innovative approach, a capsule is implanted in the eye that delivers a steady stream of growth factors to protect damage to the optic nerve, which could possibly improve vision in patients with glaucoma.

Dr. Jeffrey Goldberg
2016
First-ever creation and treatment of retinal ganglion cells using adult skin cells.
Grantee Jason Meyer, PhD, and colleagues reach a major milestone by creating and treating retinal ganglion cells (RGCs) derived from the skin cells of people with glaucoma. Healthy RGCs are crucial for vision, and this method could lead to treatments that prevent vision loss in glaucoma better than pressure-lowering treatments alone.
Watch a video featuring Dr. Meyer explaining his research.

Dr. Jason Meyer presents his research at BrightFocus’ gala in June 2022.
2016
First-ever creation and treatment of retinal ganglion cells using adult skin cells.
2017
New class of glaucoma medication available.
The launch of Rhopressa, the first approved rho-associated protein kinase (ROCK) inhibitor—fueled by early BrightFocus-funded research—represents the first entirely new class of glaucoma medication to become available in more than two decades, since the first prostaglandin analog drug was approved in 1996. Early work by BrightFocus grantee Haiyan Gong, MD, PhD, proved this class of drugs could relieve eye pressure.

Dr. Haiyan Gong
2017
First citizen science online game speeds up Alzheimer’s research.
To accelerate Alzheimer’s research, BrightFocus grantee Pietro Michelucci, PhD, created the first-ever citizen science online game Stall Catchers, where participants look at videos from the brains of mice and try to identify vessel blockages, or “stalls,” to help researchers at Cornell University. Scientists have pinpointed the link between stalls and Alzheimer’s disease in mouse models and have successfully reversed some symptoms by reducing the number of stalls. While documenting these stalls is very time-consuming for researchers, citizen scientists playing the BrightFocus-funded Stall Catchers have drastically increased research output.
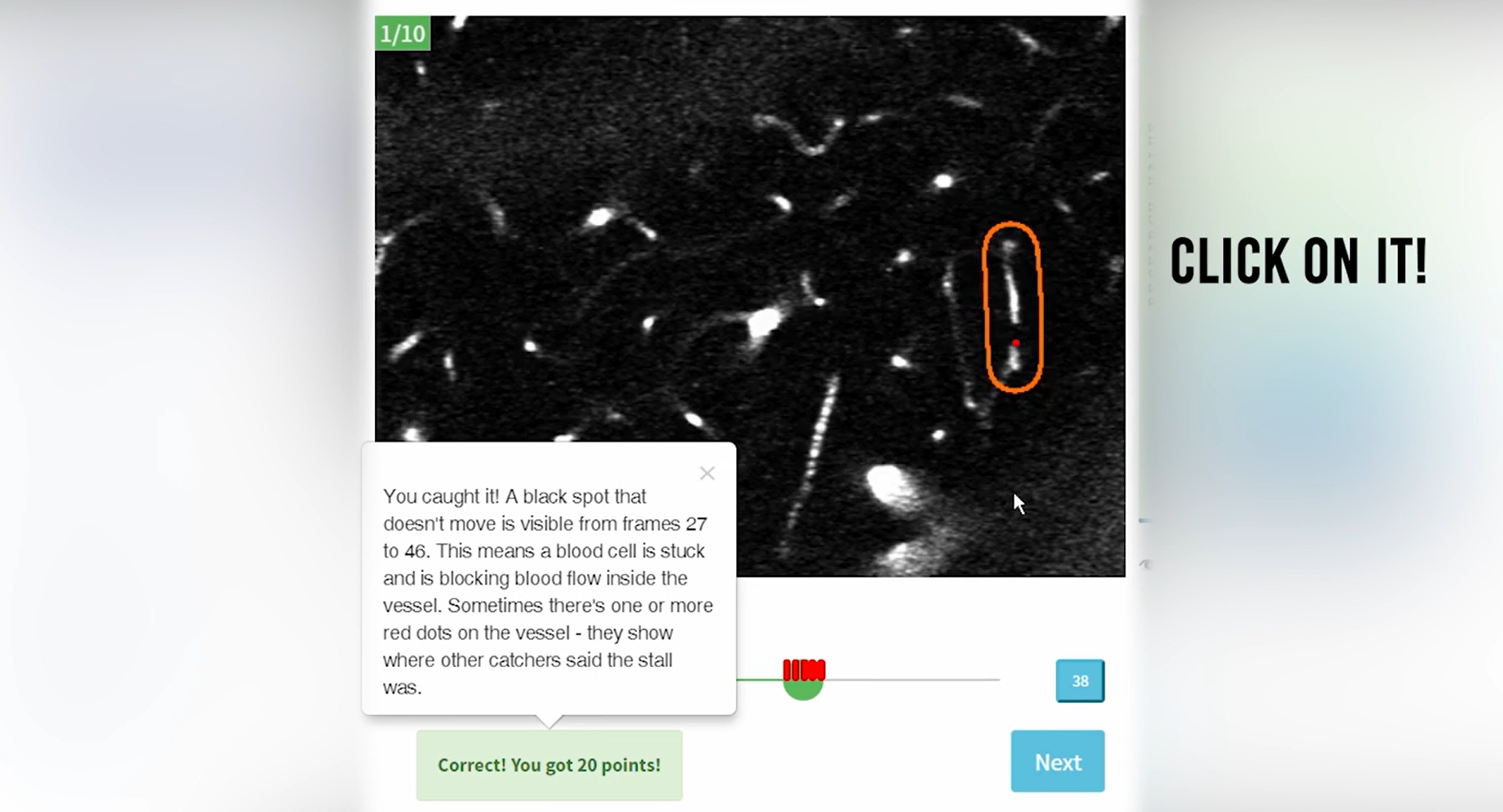
The online game Stall Catchers accelerates Alzheimer’s research.
2017
Scientists discover genetic causes and biochemical mechanisms of two rare inherited neurodegenerative diseases.
Grantee Huda Y. Zoghbi, MD, wins a Breakthrough Prize in Life Sciences for her discoveries of the genetic causes and biochemical mechanisms of two rare inherited neurodegenerative diseases, spinocerebellar ataxia and Rett syndrome. These findings help scientists better understand other neurodegenerative and neurological diseases, including Alzheimer’s, and led Dr. Zoghbi to pursue dementia research, for which she was later awarded a BrightFocus grant.

Dr. Huda Y. Zoghbi (left) and colleague.
2017
Creation of first-ever International Brain Bank for Down Syndrome-Related Alzheimer’s Disease.
With BrightFocus support, grantee Ann-Charlotte “Lotta” Granholm-Bentley, PhD, DDS, created the first-ever International Brain Bank for Down Syndrome-Related Alzheimer’s Disease, a collaborative research network that develops and shares high-quality biological research samples and studies information about the Down syndrome population, of which as many as 80% have Alzheimer’s pathology by the time they are in their 40s and 50s.

Dr. Lotta Granholm-Bentley
2019
Sleep deprivation confirmed as a risk factor for Alzheimer’s.
In a seminal BrightFocus-funded publication on sleep and beta amyloid, coauthors and grantees Sarah Fritschi, PhD, Brendan Lucey, MD, and David Holtzman, MD, PhD, are among the first to confirm that sleep deprivation is a risk factor for—not just a consequence of—Alzheimer’s disease. The findings reveal that lack of sleep alone helps drive the disease, suggesting that good sleep habits may help preserve brain health.
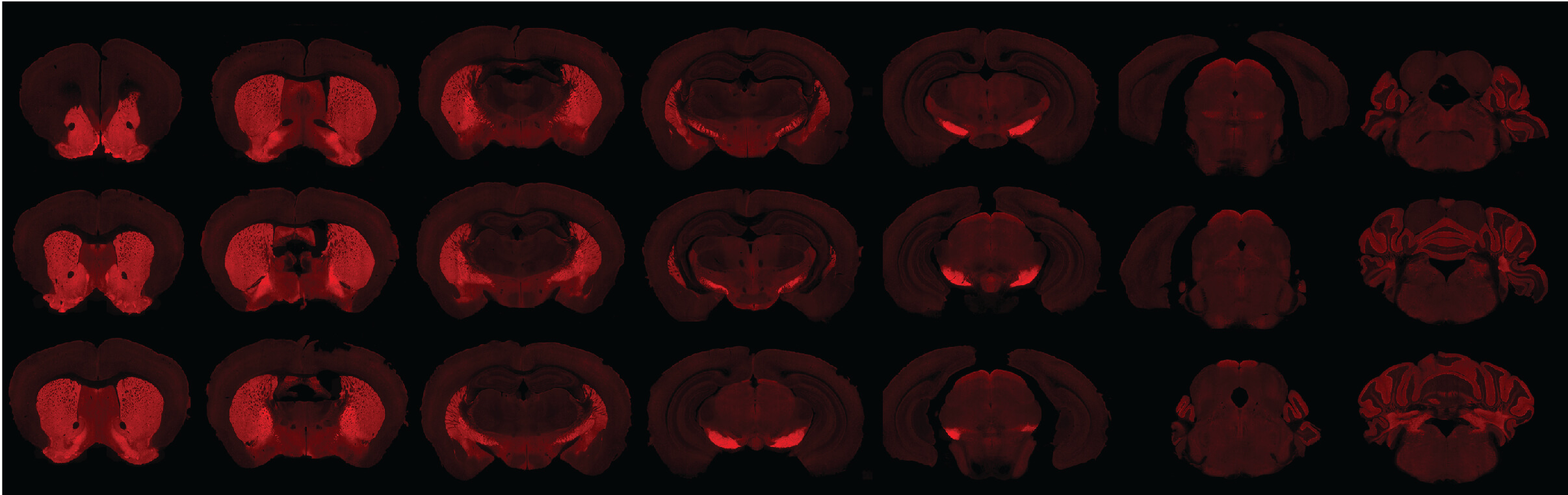
GABAergic neurons that play a critical role in sleep are pictured in red in a mouse brain. Photo courtesy of Sarah Fritschi, PhD.
2019
New Alzheimer’s model reveals genetic changes associated with cell-aging processes.
Grantee Jerome Mertens, PhD, successfully reprograms cultured skin cells from Alzheimer’s patients directly into brain neurons, creating a brain model that is not only genetically unique to each patient but also biologically “remembers” the age of the individual. This research could lead to giving Alzheimer’s neurons their own memory back, paving the way for therapeutics to prevent or treat the disease.

Dr. Jerome Mertens (second from right) and colleagues.
2020
First Alzheimer’s blood test becomes available in the U.S.
C2N Diagnostics introduces the first widely accessible blood test in the U.S. to identify early signs of Alzheimer’s disease. After receiving ritical early support from BrightFocus, the PrecivityAD blood test received an FDA “Breakthrough Device” designation, paving the way for new treatments for earlier and better patient care.

The PrecivityAD blood test identifies early signs of Alzheimer’s. Photo courtesy of C2N Diagnostics.
2020
Deep sleep protects against Alzheimer’s, new research shows.
A team including grantee Ksenia Kastanenka, PhD, finds that sleep quality may serve as a marker of future Alzheimer’s disease risk and the speed of progression. Sleep disturbance is associated with a higher rate of future beta-amyloid accumulation and deep sleep can clear toxic beta-amyloid and tau from the brain, the researchers confirm.
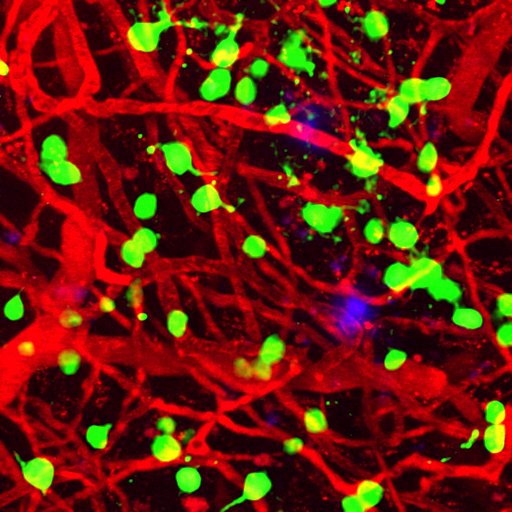
Amyloid plaques (blue) appear among brain blood vessels (red) and cortical neurons (green). Deep sleep can clear Alzheimer’s-causing amyloid plaques from the brain. Photo courtesy of Ksenia Kastanenka, PhD.
2020
Blood vessel changes in the eye may reveal early dementia.
By screening Alzheimer’s through the eye, a research team led by grantee Amir Kashani, MD, PhD, finds that changes in blood flow and vessel density in the eye may be an indicator of problems with blood circulation in the brain, signaling the risk of cognitive decline from Alzheimer’s disease and other forms of dementia. By bridging eye and brain research, BrightFocus grantees are pursuing a bold “what-if” science that could provide earlier detection and diagnosis of Alzheimer’s and other types of dementia.
Watch Dr. Kashani discuss recent research to diagnose vascular dementia through retinal scans.

Dr. Amir Kashani
2020
Blood vessel changes in the eye may reveal early dementia.
2021
Researchers develop a new wireless device to diagnose and monitor glaucoma.
BrightFocus-funded scientists including J. Crawford Downs, PhD, develop a new implantable device that could change the way glaucoma is diagnosed. The wireless device measures intraocular eye pressure and a variety of pressure parameters in the eye.

Dr. J. Crawford Downs
2021
A team of medical researchers and bioengineers, including grantee Ruchira Singh, PhD, develop the first-ever 3D cell matrix model of the human eye that replicates wet age-related macular degeneration.
The breakthrough discovery can help pinpoint what causes the disease, develop drug treatments, and determine how well drugs could work in specific patients, offering a more personalized approach to treatment.

Dr. Ruchira Singh (left) and colleague in the lab.
2021
First successful cell transplantation gives hope for new glaucoma treatment.
Researchers, including grantee Petr Baranov, MD, PhD, and team at Harvard Medical School, successfully transplant—for the first time—lab-grown retinal ganglion cells derived from induced pluripotent stem cells into the eyes of mice. Based on this work, researchers estimate that people with late-stage glaucoma could begin receiving vision-restoring transplants in the next 10 years.
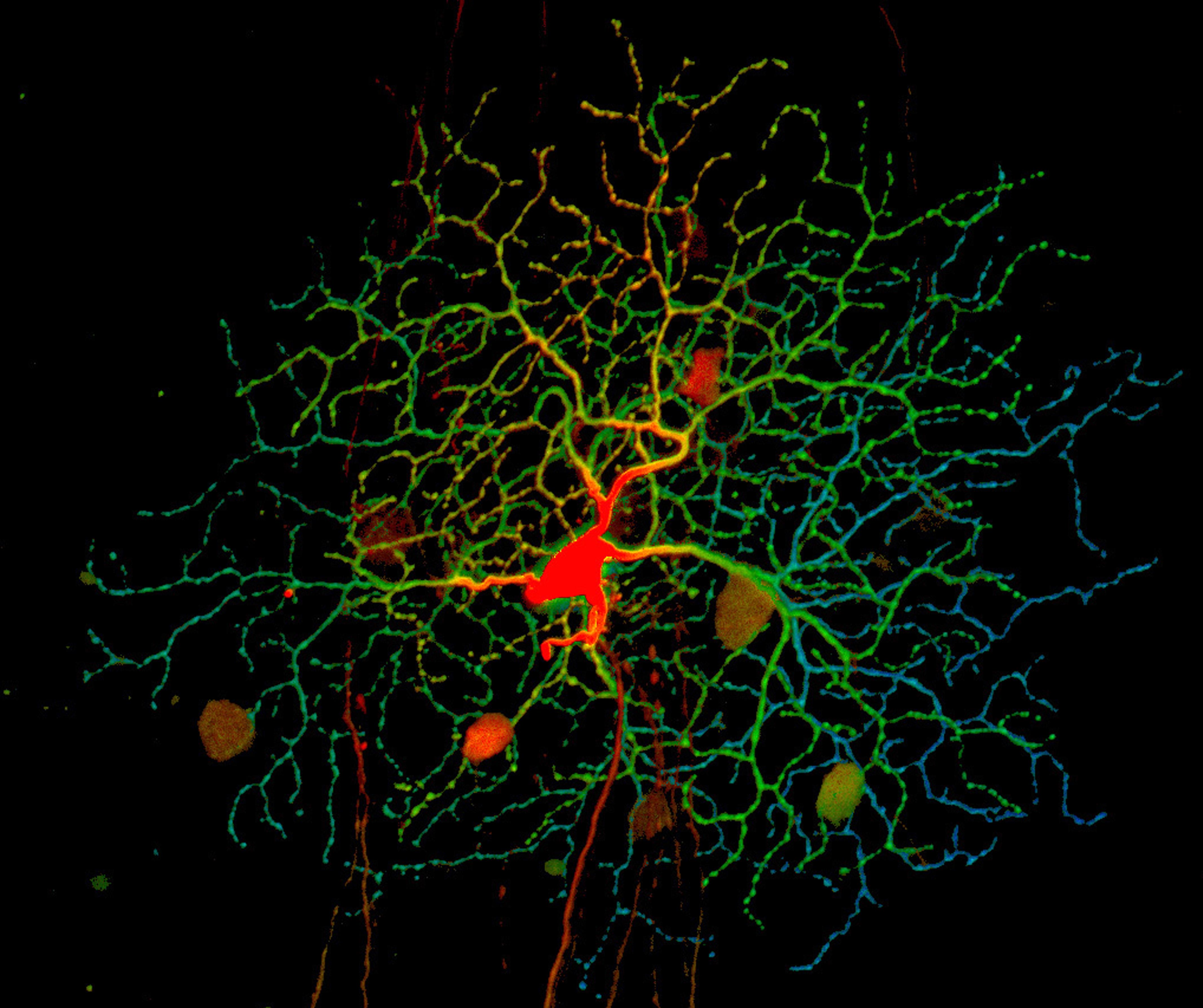
Retinal ganglion cells (blue, green, and red) receive information about the light our eyes detect from other cells in the retina at structures called dendrites (red) and then relay it to the brain via axons. Credit: National Eye Institute, National Institutes of Health (NEI/NIH)
2021
Pioneering work by grantee Ilyas Washington, PhD, leads to FDA “Breakthrough Therapy” designation for potential Stargardt disease treatment, with possibilities for treating dry AMD—two vision diseases that can lead to blindness.
BrightFocus awarded Dr. Washington one of the first research grants of his scientific career, allowing him to begin exploring a possible role for enriched vitamin A in treating vision disease.

Dr. Ilyas Washington accepts the 2022 Bench to Bedside Award at BrightFocus’ gala.
2021
Pioneering work by grantee Ilyas Washington, PhD, leads to FDA “Breakthrough Therapy” designation for potential Stargardt disease treatment, with possibilities for treating dry AMD—two vision diseases that can lead to blindness.
“BrightFocus was the first major funder of my academic lab. They gave me the opportunity – and the confidence – to believe that I could someday stop someone from losing their sight.” – Dr. llyas Washington
2021
New Alzheimer’s blood biomarker discovered.
Led by grantee Thomas Karikari, PhD, a research team identifies a less frequently measured biomarker of tau in the blood, BD-tau, that positively correlates with neurodegeneration and cognitive impairment before symptoms begin. BD-tau outperforms existing blood tests used to detect Alzheimer’s-related neurodegeneration and could lead to earlier and more accurate Alzheimer’s diagnoses.

Dr. Thomas Karikari
2021
First-ever reversal of age-related vision loss and eye damage from glaucoma in mice.
For the first time, BrightFocus grantees and Harvard scientists Bruce Ksander, PhD, and Meredith Gregory-Ksander, PhD, successfully reprogram cells in mice to reverse vision loss from glaucoma, as well as normal vision loss associated with aging. The results show strong promise of gene therapy to reprogram eye tissue to restore vision lost to glaucoma and reverse aging and age-related diseases in humans.
“Meredith and I both got our start in glaucoma research from BrightFocus grants. We’ve received significant support over the years from your Foundation, and I am absolutely certain this project would not have been possible without this generous support.”—Dr. Bruce Ksander

Meredith Gregory-Ksander, PhD, and Bruce Ksander, PhD
2021
First-ever driving test to predict Alzheimer’s disease.
Grantee Ganesh Babulal, PhD, develops a first-of-its-kind driving test that can identify Alzheimer’s disease before other symptoms appear. By evaluating driving behavior, the team detects subtle changes in cognition that might be missed by traditional testing.

A researcher from Dr. Babulal’s lab holds a device that collects data on driving behaviors in older adults that can serve as biomarkers of Alzheimer’s disease. Photo courtesy of Ganesh Babulal, PhD.
2021
Critical funding for treatment of traumatic brain injuries, which are associated with dementia later in life.
BrightFocus and the Medical Technology Enterprise Consortium jointly award $500,000 grants to researchers at Astrocyte Pharmaceuticals and a partnership between Mitochon Pharmaceuticals and the University of Kentucky for the study of neuroprotective therapeutics for the treatment of brain injuries, which could lower risk of subsequent Alzheimer’s and related dementias.
Potential therapies target traumatic brain injuries, which can lead to dementia.
2022
Historic first patient-derived stem cell therapy for dry age-related macular degeneration.
Macular Degeneration Research grantees Kapil Bharti, PhD, and Amir Kashani, MD, PhD, were part of the National Institutes of Health clinical trial team that pioneered the first patient-derived stem cell therapy for dry age-related macular degeneration. Our early research helped pave the way for this groundbreaking surgery.
Dr. Kapil Bharti (left) and Dr. Amir Kashani
2022
First-of-its-kind artificial intelligence model developed that can one day detect Alzheimer’s disease by reading a patient’s retina images.
A team of Hong Kong scientists led by grantee Carol Cheung, PhD, develops a faster way to screen for Alzheimer’s disease using an AI model that reads retina scans. This highly accurate method could enable people, who would receive their results immediately, to benefit from early intervention and treatment.
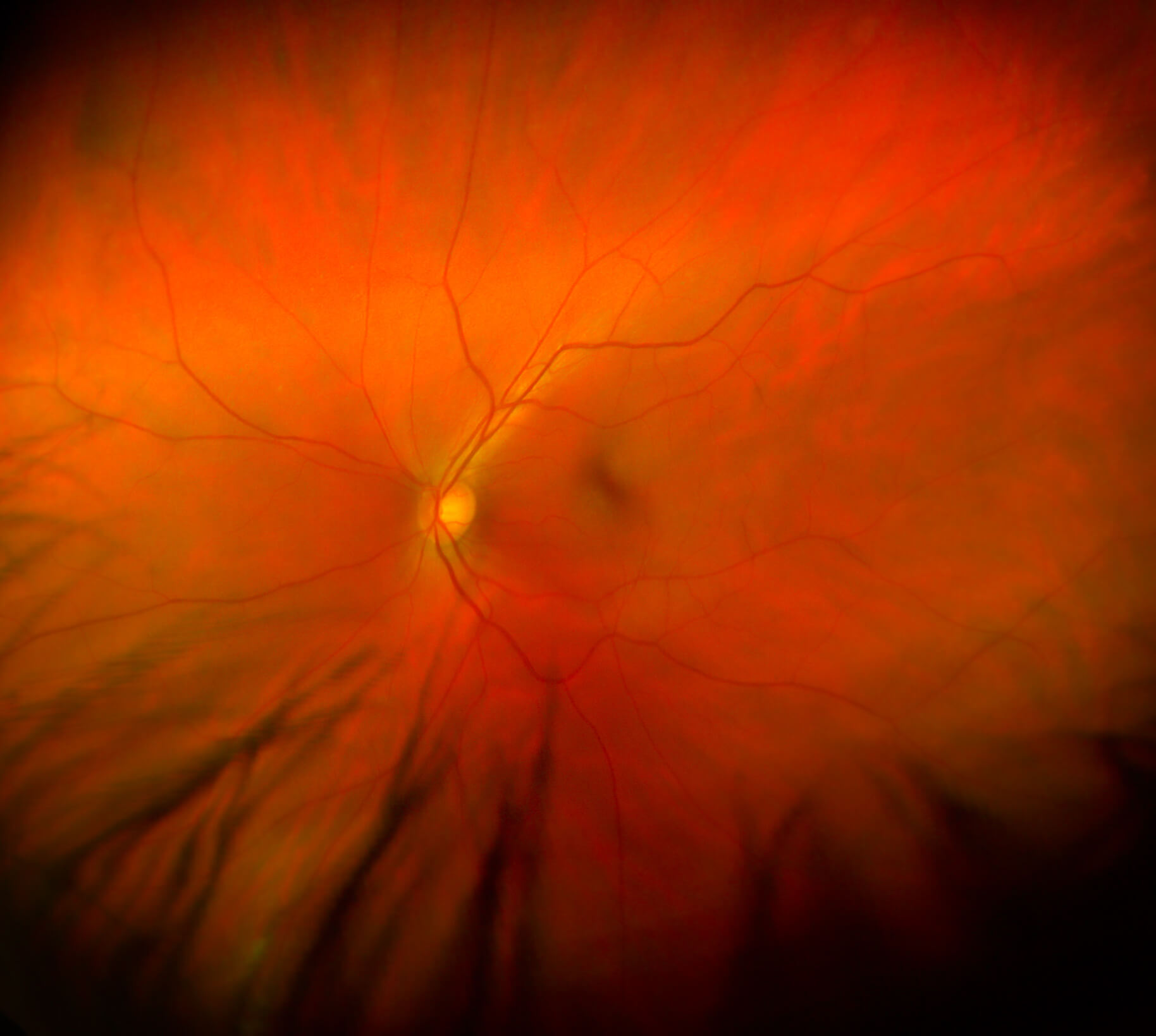
A close-up view of the retina, which could reveal critical signs of Alzheimer’s disease.
2023
FDA approves first treatment for geographic atrophy, an advanced form of age-related macular degeneration.
Key early Macular Degeneration Research-funded findings from 2006 would be pivotal for the first FDA-approved complement pathway drug to treat geographic atrophy.
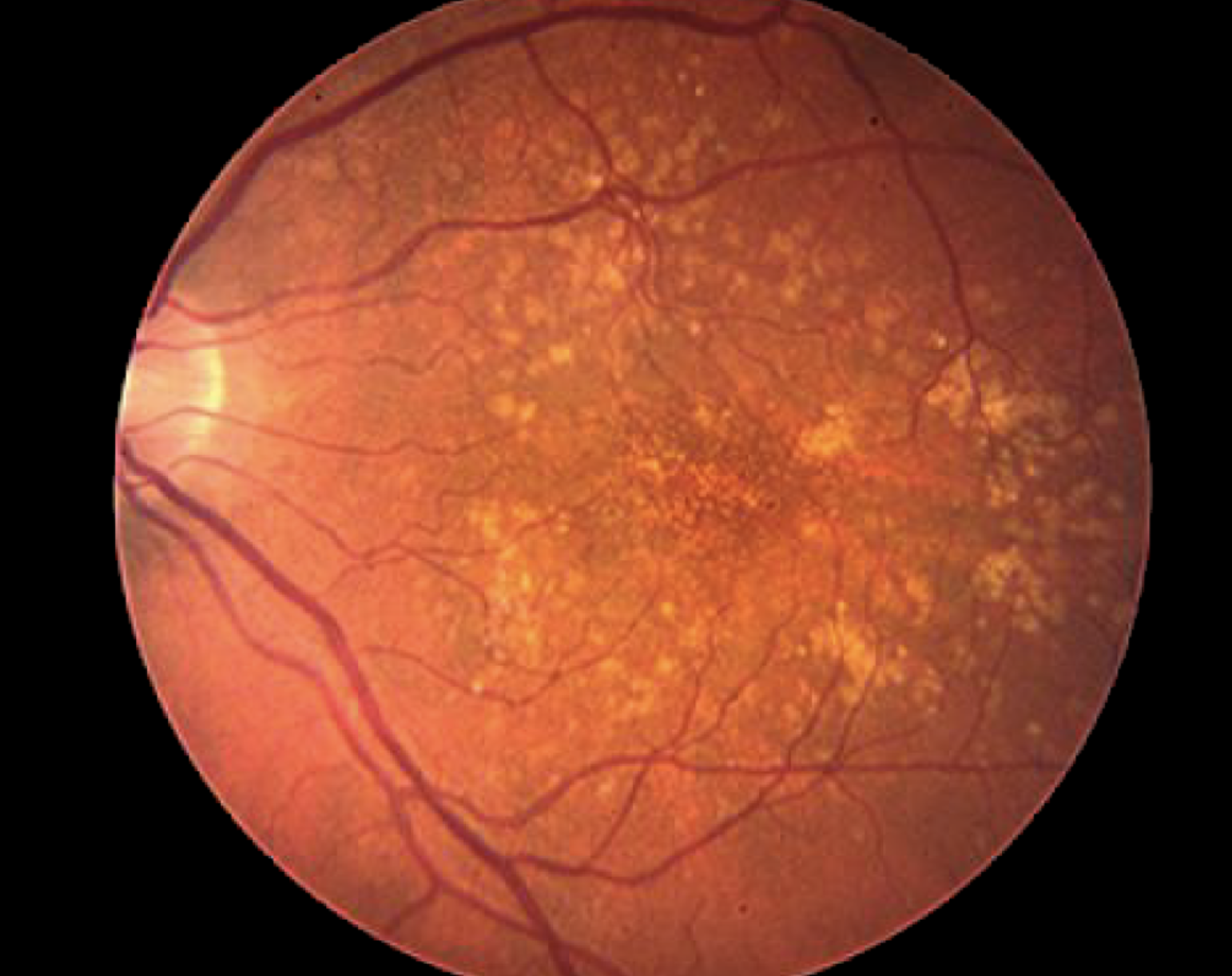
Image of an eye with geographic atrophy, an advanced and severe form of dry age-related macular degeneration.